PARAMUS, NJ: May 14, 2025 – NS Pharma, Inc. (NS Pharma), a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku) announced today that the U.S. Food and Drug Administration has accepted for review the Biologics License Application (BLA) submission by REGENXBIO Inc. (REGENXBIO; Headquarters: Rockville, Maryland, USA; CEO: Curran M. Simpson, NASDAQ: RGNX) for RGX-121 (clemidsogene lanparvovec), a potential first-in-class, investigational gene therapy for the treatment of Mucopolysaccharidosis II (MPS II). The FDA granted REGENXBIO’s BLA Priority Review with a Prescription Drug User Fee Act (“PDUFA”) target action date of November 9, 2025.
In January 2025, Nippon Shinyaku and REGENXBIO entered into a strategic partnership for the development and commercialization of RGX-121, as well as RGX-111, which is for the treatment of MPS I. Upon potential approval of RGX-121, NS Pharma will be exclusively responsible for commercializing RGX-121 in the U.S.
“This FDA decision represents a significant milestone in bringing a new, potentially life-changing treatment option to patients in the MPS community,” said NS Pharma President, Yukiteru Sugiyama, Ph.D. “We are excited about our partnership with REGENXBIO and the value of our combined expertise in generating renewed hope for MPS families.”
For more details, please see the press release from REGENXBIO: https://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-fda-acceptance-and-priority-review-bla-rgx
About RGX-121 (clemidsogene lanparvovec)
RGX-121 is a potential one-time AAV therapeutic for the treatment of boys with MPS II, designed to deliver the iduronate-2-sulfatase (IDS) gene to the central nervous system (CNS). Delivery of the IDS gene within cells in the CNS could provide a permanent source of secreted iduronate-2-sulfatase (I2S) protein beyond the blood-brain barrier, allowing for long-term cross correction of cells throughout the CNS. RGX-121 expressed protein is structurally identical to normal I2S.
RGX-121 has received Orphan Drug Product, Rare Pediatric Disease, Fast Track and Regenerative Medicine Advanced Therapy designations from the FDA.
About MPS II
MPS II, or Hunter Syndrome, is a rare, X-linked recessive disease caused by a deficiency in the lysosomal enzyme I2S leading to an accumulation of glycosaminoglycans (GAGs), including heparan sulfate (HS) in tissues which ultimately results in cell, tissue, and organ dysfunction, including in the CNS. In severe forms of the disease, early developmental milestones may be met, but developmental delay is readily apparent by 18 to 24 months. Specific treatment to address the neurological manifestations of MPS II remains a significant unmet medical need. Key biomarkers of I2S enzymatic activity in MPS II patients include its substrate heparan sulfate (HS) D2S6, which has been shown to correlate with neurocognitive manifestations of the disorder.
About REGENXBIO Inc.
REGENXBIO is a leading clinical-stage biotechnology company seeking to improve lives through the curative potential of gene therapy. Since its founding in 2009, REGENXBIO has pioneered the development of AAV Therapeutics, an innovative class of gene therapy medicines. For more information, please visit www.regenxbio.com.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku Co., Ltd. For more information, please visit nspharma.com.
U.S. Media Contact:
media@nspharma.com
PARAMUS, NJ: April 18, 2025 – NS Pharma, Inc. (NS Pharma) a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced today that the U.S Food & Drug Administration (FDA) has granted Orphan Drug Designation to NS-229, which is being developed for the treatment of the rare disease eosinophilic granulomatosis with polyangiitis (EGPA). NS-229 is being investigated as a selective Janus kinase 1 (JAK1) inhibitor to help regulate immune cell function and prevent the immune system from causing tissue damage.
FDA Orphan Drug Designation status is granted for treatments of rare diseases affecting fewer than 200,000 people in the U.S. This designation provides NS Pharma with a seven-year market exclusivity period, in support of the company’s continued development and evaluation of this therapy.
About EGPA
EGPA is a rare autoimmune disease causing inflammation in the small-to-medium-sized blood vessels which can cause tissue and organ damage to the lungs, sinuses, peripheral nerves, skin, and kidneys. EGPA is generally preceded by symptoms of bronchial asthma and allergic rhinitis. The cause is unknown. It is estimated that EGPA affects between 5,600 and 14,500 people in the U.S.*
“There are several factors associated with the inflammatory response in EGPA that could be regulated by JAK1,” explained NS Pharma Vice President, Research & Development, Takeshi Seita. “Our therapy has been designed to target this specific enzyme.”
A Phase 2 global study of NS-229 is being conducted by Nippon Shinyaku and NS Pharma.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku group of companies. For more information, please visit nspharma.com.
U.S. Media Contact:
media@nspharma.com
*Estimated prevalences 1.7 / 100,0001) and 4.4 / 100,0002) were multiplied by a 2023 U.S. population estimate of around 330 million and rounded to nearest hundred.
1) Bell, CF., Lau, M., Shen, Q. Clinical and Economic Characteristics of Patients Diagnosed with Eosinophilic Granulomatosis with Polyangiitis (EGPA, formerly Churg-Strauss Syndrome) in the United States [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9).
2) Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The Epidemiology of Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis in Olmsted County, Minnesota: A Twenty-Year US Population-Based Study. Arthritis Rheumatol. 2017;69:2338-2350
PARAMUS, NJ: March 26, 2025 – NS Pharma, Inc. (NS Pharma), a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced today that Capricor Therapeutics, Inc. (Headquarters: California, USA, CEO: Linda Marbán, NASDAQ: CAPR) has recently released positive long-term data from its ongoing HOPE-2 open label extension (“OLE”) clinical trial showing the potential of deramiocel to slow disease progression and preserve upper limb function in patients with Duchenne muscular dystrophy (“DMD”).
Nippon Shinyaku and Capricor entered into an exclusive distribution agreement for deramiocel for the U.S. in January 2022. NS Pharma will be exclusively responsible for commercialization and distribution of deramiocel in the U.S.
For full details, see the Capricor press release here.
About Deramiocel
Deramiocel consists of allogeneic cardiosphere-derived cells (“CDCs”), a rare population of cardiac cells that have been shown in preclinical and clinical studies to exert potent immunomodulatory and anti-fibrotic actions in preservation of cardiac and skeletal muscle function in dystrophiopathies such as DMD. CDCs act by secreting extracellular vesicles known as exosomes, which target macrophages and alter their expression profile to adopt a healing, rather than a pro-inflammatory phenotype. CDCs have been the subject of over 200 peer-reviewed scientific publications and have been administered to over 250 human subjects across several clinical trials.
About Duchenne Muscular Dystrophy (Duchenne)
Duchenne is a form of muscular dystrophy that occurs primarily in males. It causes progressive weakness and loss of skeletal, cardiac, and respiratory muscles. Early signs of Duchenne may include delayed ability to sit, stand or walk. There is a progressive loss of mobility, and by adolescence, patients with Duchenne may require the use of a wheelchair. Cardiac and respiratory muscle problems begin in the teenage years and lead to serious, life-threatening complications. For more information about Duchenne, please visit wespeakduchenne.com.
About Capricor Therapeutics, Inc.
Capricor (NASDAQ: CAPR) is a biotechnology company dedicated to advancing transformative cell and exosome-based therapeutics to redefine the treatment landscape for rare diseases. For more information, https://www.capricor.com.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku Co., Ltd. For more information, please visit nspharma.com.
US Media Contact:
media@nspharma.com
PARAMUS, NJ: March 6, 2025 – NS Pharma, Inc. (NS Pharma), a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced today that acceptance has been received by Capricor Therapeutics, Inc. (Headquarters: California, USA, CEO: Linda Marbán, NASDAQ: CAPR) from the U.S. Food and Drug Administration (FDA) for the Biologics License Application (BLA) filing for deramiocel, an investigational cell therapy, as a treatment for patients diagnosed with Duchenne muscular dystrophy (“DMD”) cardiomyopathy. The FDA granted the BLA Priority Review with a Prescription Drug User Fee Act (“PDUFA”) target action date of August 31, 2025, and at this time, the FDA has not identified any potential review issues.
Nippon Shinyaku and Capricor entered into an exclusive distribution agreement for deramiocel for the U.S. in January 2022. NS Pharma will be exclusively responsible for commercialization and distribution of deramiocel in the U.S.
“Deramiocel has the potential to address a clear, unmet medical need for patients diagnosed with DMD,” said NS Pharma President, Yukiteru Sugiyama, Ph.D. “We are excited for the possibility to bring additional treatment options and renewed hope to families of the rare disease community.”
For more details, please see the press release from Capricor.
https://www.capricor.com/investors/news-events/press-releases/detail/305/capricor-therapeutics-announces-fda-acceptance-and-priority
About Deramiocel
Deramiocel consists of allogeneic cardiosphere-derived cells (“CDCs”), a population of stromal cells that have been shown in preclinical and clinical studies to exert potent immunomodulatory, antifibrotic and regenerative actions in dystrophinopathy and heart failure. CDCs act by secreting extracellular vesicles known as exosomes, which target macrophages and alter their expression profile so that they adopt a healing, rather than a pro-inflammatory, phenotype. CDCs have been the subject of over 100 peer-reviewed scientific publications and have been administered to over 200 human subjects across several clinical trials.
About Duchenne Muscular Dystrophy (Duchenne)
Duchenne is a form of muscular dystrophy that occurs primarily in males. It causes progressive weakness and loss of skeletal, cardiac, and respiratory muscles. Early signs of Duchenne may include delayed ability to sit, stand or walk. There is a progressive loss of mobility, and by adolescence, patients with Duchenne may require the use of a wheelchair. Cardiac and respiratory muscle problems begin in the teenage years and lead to serious, life-threatening complications. For more information about Duchenne, please visit wespeakduchenne.com.
About Capricor Therapeutics, Inc.
Capricor (NASDAQ: CAPR) is a biotechnology company dedicated to advancing transformative cell and exosome-based therapeutics to redefine the treatment landscape for rare diseases. Capricor is also harnessing the power of its exosome technology, using its proprietary StealthX™ platform in preclinical Capricor (NASDAQ: CAPR) is a biotechnology company dedicated to advancing transformative cell and exosome-based therapeutics to redefine the treatment landscape for rare diseases. Capricor is also harnessing the power of its exosome technology, using its proprietary StealthX™ platform in preclinical development focused on the areas of vaccinology, targeted delivery of oligonucleotides, proteins and small molecule therapeutics to potentially treat and prevent a diverse array of diseases. For more information, https://www.capricor.com.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku Co., Ltd. For more information, please visit nspharma.com.
US Media Contact:
media@nspharma.com
In rare disease research, collaboration is often the catalyst that transforms a promising idea into a life-changing treatment.
It’s this approach that allows us to develop life-changing medicines to treat rare diseases. As NS Pharma Vice President, Innovation Research Partnering Tatsuaki Morokata, Ph.D., puts it, “Collaborating with others acts as an accelerating system. A good collaboration enables us to do together what otherwise cannot be done.”

Tatsuaki Morokata, PhD, MBA
Vice President, Innovation Research Partnering, NS Pharma
Our expertise in pharmacology, clinical development, and regulatory affairs enables us to swiftly progress therapies from concept to clinic. Now, through our Innovation Research Partnering division, we’re forming strategic alliances with cutting-edge companies to turn pioneering science into real-world solutions.
One such partnership is with MiNA Therapeutics, the world leader in RNA activation (RNAa) drugs, revolutionary medicines that boost gene function. In April 2024, Nippon Shinyaku, Co., Ltd. (“Nippon Shinyaku”), the parent company of NS Pharma, entered a joint research agreement with MiNA Therapeutics to develop small activating RNAs (saRNAs) to treat rare and intractable diseases of the central nervous system (CNS) by RNAa.
The mission of NS Pharma is to push the boundaries of nucleic acid therapies1 with innovative drug discovery modalities. MiNA Therapeutics shares this goal, using pioneering RNAa medicines to target what was previously deemed “undruggable” by conventional approaches.
MiNA’s proprietary RNAa technology powers efficient saRNA synthesis and screening, while NS Pharma’s pharmacological expertise drives the drug development process. Co-creation combines these strengths, accelerating the delivery of high-quality candidates.
| The Challenge
Around 95% of rare diseases lack approved treatments.2 Conventional approaches, including gene therapy, often fail, particularly when targeting longer genes (>4.7 kb). |
The Strategy
RNAa therapeutics unlock possibilities for targets once deemed untreatable. We’re using this powerful technology to tackle severe neurological disorders. |
The Vision
Collaboration drives unique, effective solutions at unparalleled speed. By combining our strengths, we’re accelerating drug development to help patients sooner. |
Our alliance with MiNA focuses on enhancing gene expression in rare neurological disorders through cutting-edge RNAa therapeutics. The initial target of this collaboration is a severe neurodevelopmental disorder caused by a single defective gene. Although estimated to affect hundreds of thousands of people worldwide, there are currently no approved treatments for this condition.
This disorder is caused by mutations that result in the loss of function of a gene, leading to insufficient levels of the corresponding protein. saRNAs offer a novel way to address this by enhancing the affected gene’s expression, potentially restoring normal function.3 “MiNA’s agility and complementary expertise will enable us to bring this product to the clinic much faster, ultimately helping patients sooner,” Morokata emphasizes.
| 80%
Of rare diseases have a genetic cause2 Around 240 million people worldwide have a rare genetic disorder, with most lacking approved treatments. We’re expediting nucleic acid drug development to close this treatment gap. |
⬆2-10
Fold increase in gene expression4 RNAa therapeutics provide a game-changing opportunity to treat loss-of-function genetic disorders. These drugs restore physiological protein levels by substantially increasing endogenous gene expression. |
⬇50%
Decrease in candidate development timeline The complementary combination of MiNA’s systematic drug discovery approach and NS Pharma’s efficient clinical development process drastically reduces the time needed to produce a clinical candidate. |
With RNAa technology, we can upregulate genes underlying loss-of-function disorders that cause developmental, motor, and behavioral delays, offering solutions where other therapies have failed.
RNAa therapeutics directly tackle loss-of-function disorders by boosting natural gene expression, increasing it by 2 to 10 times.4 They uniquely activate challenging gene types, including large genes and those silenced epigenetically.
By activating endogenous gene expression, RNAa drugs achieve more physiologically relevant protein expression levels. This mitigates risks associated with overexpression or the permanent introduction of foreign genetic material. The highly specific nature of RNAa therapeutics also minimizes the potential for off-target effects.
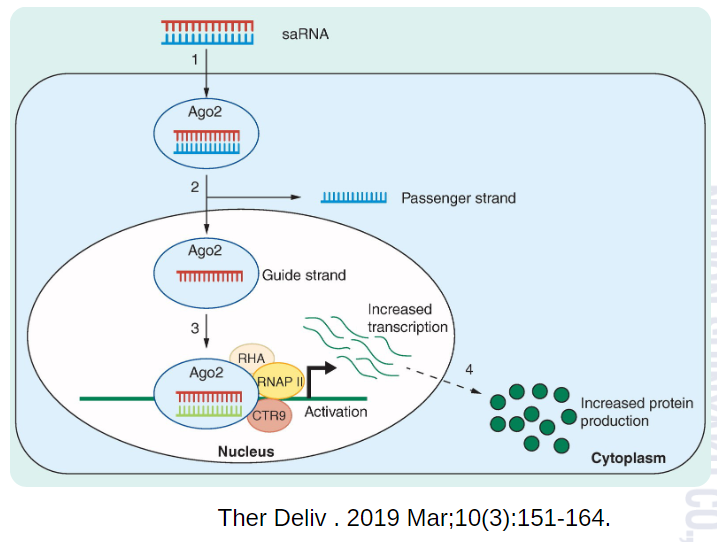
Combining NS Pharma’s robust development pipeline with MiNA’s cutting-edge RNAa technology positions us to make significant strides in areas where traditional therapies have struggled.
As we look to the future, our collaboration with MiNA Therapeutics represents more than just a strategic partnership—it embodies our commitment to advancing the treatment of rare diseases through co-creation and innovation.
We are committed to finding solutions for the unmet medical needs of patients worldwide. “By combining our strengths and resources, we can compete with very large pharmaceutical companies, particularly in the rare disease space,” Morokata explains. With our deep expertise and patient-centric focus, we are eager to partner with like-minded organizations to bring groundbreaking treatments to those in need.
Together, we can make a difference.
>Partner with us to advance rare disease treatment<
References
At NS Pharma, leadership is more than just a role—it’s a commitment to empowering teams, fostering innovation, and building strong partnerships that push the boundaries of rare disease treatments.
The newest member of our executive team, Don Foy, embodies this approach. With over 24 years of pharma experience—including 17 in leadership roles—Don brings a wealth of expertise in sales, marketing, market access, and commercial strategy to his role as head of Commercial. This expertise is grounded in a leadership style that prioritizes trust, collaboration, and professional development.

Don Foy, Head of Commercial, NS Pharma
Trust as the Cornerstone of Leadership
Shinrai is the Japanese word for trust, a core value that shapes every aspect of our work at NS Pharma. For Don, building trust through integrity is not just a professional value but a personal one, rooted in lessons he learned from his father.
He remembers how his father, an association executive, led with empathy, care, and a deep commitment to his team’s well-being: “I observed how he treated and led people, and when he retired, the accolades that he received from his staff were quite overwhelming,” Don recalls.
This early lesson has shaped Don’s belief that great leadership comes from genuinely caring for those you lead: “Trust is the foundation for everything, be it internal or external relationships,” he explains. “As a leader, you have to demonstrate care and concern for the people you serve. They also need to see your vulnerabilities. The more you can do that, the more you connect and build trust with your people.”
For Don and the rest of NS Pharma’s executive team, trust built on transparency and integrity is the critical element that fosters collaboration, enhances performance, and creates lasting partnerships. Shinrai isn’t just an internal value at NS Pharma—it shapes our relationships with healthcare professionals, industry partners, and the patients we serve.

[Don with his father]
Collaboration accelerates drug development. At NS Pharma, the Commercial team is pivotal in maintaining strategic partnerships that allow us to bring life-changing therapies to patients with rare diseases.
A prime example is our ongoing collaboration with Capricor Therapeutics to launch deramiocel (CAP-1002), a new cell therapy product for Duchenne muscular dystrophy. Don’s team ensures seamless execution at every step of the partnership—from planning to launch. This starts with aligning internal and external stakeholders through open communication and mutual accountability.
According to Don, successful partnerships hinge on three fundamental principles:
This hands-on approach enables NS Pharma’s Commercial team to be proactive, adapt swiftly to meet challenges, and ensure complete alignment—keeping the focus on delivering safe and effective therapies to patients as quickly as possible.
Our extensive commercial infrastructure is one of our greatest strengths, providing an invaluable advantage to our partners. With cross-continental reach and a finely tuned distribution network, we deliver an efficient, scalable platform to drive sales and maximize market reach.
The Commercial team supports this infrastructure, with senior directors overseeing patient services, market access, sales, marketing, and operations. “Our senior directors coordinate with consultants, healthcare professionals, patients, payers, advocacy organizations, and other groups, keeping us aligned to ensure we successfully accomplish our commercial objectives,” Don explains.
Through NS Pharma’s strategic leadership, cross-departmental coordination, and robust international sales infrastructure, our partners can confidently focus on innovation, knowing they have a dependable partner for commercialization success.
>Partner with us to advance rare disease treatment<
Shaping the Future Together
Under Don’s leadership, the Commercial team continues to build strong partnerships, empower future leaders, and drive the development of groundbreaking therapies that make a real difference in the lives of patients with rare diseases. “To be able to manage Viltepso, a four-year-old product in the marketplace, and the opportunity to launch an exciting new product in cell therapy,” remarks Don. “What an exciting time to be at NS Pharma.”
We couldn’t agree more. Whether you’re looking to partner with NS Pharma or join our growing team, we invite you to be part of the exciting breakthroughs shaping the future of rare disease treatment. Together, guided by the principles of Shinrai and a shared commitment to improving patient outcomes, we’re building a future that brings hope and healing to those who need it most.
Transform the future of rare disease treatment with us.
KYOTO, Japan, January 27, 2025 – Nippon Shinyaku Co., Ltd. (Headquarters: Kyoto, Japan, President: Toru Nakai) announced that Nippon Shinyaku and AB2 BIO Ltd. (Headquarters: Lausanne, Switzerland, CEO: Djordje Filipovic) have entered into an option agreement for the rights of commercialization of Tadekinig alfa for the treatment of NLRC4 mutation and XIAP deficiency in the U.S. by Nippon Shinyaku. AB2 BIO Ltd. is developing Tadekinig alfa for the treatment of NLRC4 mutation and XIAP deficiency in the U.S. and Europe.
NLRC4 mutation and XIAP deficiency are types of rare and serious hereditary autoinflammatory diseases, and elevated levels of interleukin 18 (IL-18) cause a variety of inflammatory symptoms. Most patients show symptoms in infancy and the inflammation persists throughout their lives. The number of patients with both NLRC4 mutation and XIAP deficiency is small, and the long-term prognosis is not clear, but these are serious diseases that make it difficult for many patients to reach adulthood. There are no drugs on the market for the treatment of either of these diseases, and new effective treatments are needed.
Tadekinig alfa is a recombinant human IL-18 binding protein, and it suppresses IL-18 related immune and inflammatory responses by specifically binding to excessively produced IL-18. Tadekinig alfa has received Orphan Drug Designation, Breakthrough Therapy Designation and Rare Pediatric Disease Designation in the U.S., and Orphan Drug Designation in Europe. In addition, AB2 BIO Ltd. has completed Phase III trials in North America and Europe, and is currently preparing for submission of a Biologics License Application (BLA) in the U.S.
After Nippon Shinyaku exercises its option rights and AB2 BIO Ltd. obtains the BLA approval in the U.S., NS Pharma, Inc. (New Jersey, USA, President: Yukiteru Sugiyama), a wholly owned subsidiary of Nippon Shinyaku, will market Tadekinig alfa.
Nippon Shinyaku is working to develop new treatments for intractable and rare diseases and aims to be a company that is trusted by society through the creation of unique drugs.
About Nippon Shinyaku
Based on Nippon Shinyaku’s business philosophy, “Helping people lead healthier, happier lives,” we aim to be an organization trusted by the community through creating unique medicines that will bring hope to patients and families suffering from illness. Please visit our website (https://www.nippon-shinyaku.co.jp/english/) for products or detailed information.
About AB2 BIO Ltd.
AB2 Bio is a biotech company developing innovative therapies for the treatment of severe systemic autoinflammatory diseases and conditions driven by IL-18. The company is advancing Tadekinig alfa in a wide range of IL-18 mediated hyperinflammatory diseases and conditions, including rare orphan diseases with high unmet medical needs, at clinical and pre-clinical phase.
AB2 Bio was founded in 2010 and is headquartered in the Innovation Park at the Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland. More information can be found on http://www.ab2bio.com.
Contact
Corporate Communications Dept., Nippon Shinyaku Co., Ltd.
e_mail_kouhou@po.nippon-shinyaku.co.jp
KYOTO, Japan, January 14, 2025 – Nippon Shinyaku Co., Ltd. (Nippon Shinyaku; Headquarters: Kyoto; President, Toru Nakai) announced that Nippon Shinyaku and REGENXBIO Inc. (REGENXBIO; Headquarters: Rockville, Maryland, USA; CEO: Curran M. Simpson, NASDAQ: RGNX) have entered into an exclusive license agreement for RGX-121 and RGX-111 for the treatment of Mucopolysaccharidosis II and I (MPS II and I), respectively. Under the terms of the licensing agreement, Nippon Shinyaku will receive exclusive commercialization rights in the United States (U.S.) and exclusive development and commercialization rights in Asia including Japan, and REGENXBIO will retain commercial rights in the rest of the world. After approval of the Biologics License Application in the U.S., RGX-121 and RGX-111 will be marketed by NS Pharma, Inc. (New Jersey, USA; President: Yukiteru Sugiyama), a wholly owned subsidiary of Nippon Shinyaku, in the U.S.
Mucopolysaccharidosis (MPS) is a congenital metabolic disorder in which a specific enzyme is defective or inactive due to genetic factors, resulting in the accumulation of specific glycosaminoglycans (“GAGs”), a type of mucopolysaccharide, and is classified into several forms according to the gene responsible for the disease. The accumulation of GAGs causes systemic organ damage, including the central nervous system, in severe cases, and the prognosis is 10 to 15 years of age. Currently, there is no curative treatment for the disease, and the mainstay of treatment is the suppression of progression through enzyme replacement therapy.
RGX-121 and RGX-111 are first-in-class, investigational gene therapies for the treatment of MPS II and MPS I, respectively. For RGX-121, REGENXBIO has received Fast Track Designation, Rare Pediatric Disease Designation, Regenerative Medicine Advanced Therapy Designation, and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA). Submission of a rolling Biologics Licensing Application (BLA) for RGX-121 is ongoing. For RGX-111, REGENXBIO has received Fast Track Designation, Rare Pediatric Disease Designation, and Orphan Drug Designation from the FDA and has been conducting a Phase I/II trial in the U.S., Brazil, and Israel.
Nippon Shinyaku is focusing on the field of intractable, rare disorders. We expect that RGX-121 and RGX-111 will contribute to the treatment of patients suffering from MPS.
Entry into force of this agreement is subject to completion of review under the Hart-Scott-Rodino (HSR) Antitrust Reform Act in the U.S.
About MPS II
MPS II, also called Hunter syndrome, is a rare, congenital metabolic disorder caused by a deficiency in the iduronate-2-sulfatase, one of the enzymes that degrades glycosaminoglycans (GAGs). The lack of this enzyme causes heparan sulfate and dermatan sulfate to accumulate in all body tissues. When it develops, it causes systemic symptoms such as growth retardation, osteoarticular symptoms, valvular heart disease, and central nervous system disorders. The current treatment for this disorder is palliative care, enzyme replacement therapy (ERT) and bone marrow and stem cell transplantation.
About MPS I
MPS I is a congenital dysmetabolic disease caused by congenital deficiency or reduced activity of alpha-L-isuronidase, one of the enzymes that degrades glycosaminoglycans (GAGs) in steps, resulting in intracellular accumulation of dermatan sulfate and heparan sulfate and damage to multiple organs. MPS I is classified into two types: severe, with intellectual disability, which is also called Hurler’s syndrome and mild.
About HSR Antitrust Reform Act of 1976
The HSR Act provides that before certain size of mergers, tender offers or other acquisition transactions (including certain grants of executive compensation) may be completed, both parties must file notification forms with the Antitrust Division of the Department of Justice and the Federal Trade Commission, and a statutory waiting period expires or is terminated. The HSR Act has been expanded for pharmaceutical licensing agreements in 2013.
About Nippon Shinyaku
Based on Nippon Shinyaku’s business philosophy, “Helping people lead healthier, happier lives,” we aim to be an organization trusted by the community through creating unique medicines that will bring hope to patients and families suffering from illness.
Please visit our website (https://www.nippon-shinyaku.co.jp/english/) for products or detailed information.
About REGENXBIO Inc.
REGENXBIO is a leading clinical-stage biotechnology company seeking to improve lives through the curative potential of gene therapy. Since its founding in 2009, REGENXBIO has pioneered the development of AAV Therapeutics, an innovative class of gene therapy medicines. For more information, please visit www.regenxbio.com.
Contact
Corporate Communications Dept., Nippon Shinyaku Co., Ltd.
e_mail_kouhou@po.nippon-shinyaku.co.jp
PARAMUS, NJ: January 13, 2025 – NS Pharma, Inc. (NS Pharma), a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced that the National Center of Neurology and Psychiatry (NCNP) has published in the journal Cell Reports Medicine the results of an investigator-initiated clinical trial (first in human trial) of NS-089/NCNP-02 (brogidirsen), which is being developed by Nippon Shinyaku for the treatment of Duchenne muscular dystrophy (Duchenne). A global Phase II study of NS-089/NCNP-02 is being conducted by Nippon Shinyaku and NS Pharma. Please check the press release from NCNP for a summary of the paper. Additionally, the paper is available under open access here.
NS-089/NCNP-02 is an antisense oligonucleotide co-discovered by Nippon Shinyaku and NCNP and is expected to be a therapeutic drug for Duchenne patients who have dystrophin gene mutations amenable to exon 44 skipping.
NS Pharma has been actively working to develop agents for the treatment of intractable and rare diseases, with a goal of launching treatments for patients with Duchenne as soon as possible.
About Duchenne Muscular Dystrophy (Duchenne)
Duchenne is a progressive form of muscular dystrophy that occurs primarily in males. It causes progressive weakness and loss of skeletal, cardiac, and respiratory muscles. Early signs of Duchenne may include delayed ability to sit, stand or walk. There is a progressive loss of mobility, and by adolescence, patients with Duchenne may require the use of a wheelchair. Cardiac and respiratory muscle problems begin in the teenage years and lead to serious, life-threatening complications. For more information about Duchenne, please visit wespeakduchenne.com.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku group of companies. For more information, please visit nspharma.com.
U.S. Media Contact:
media@nspharma.com
PARAMUS, NJ: November 26, 2024 – NS Pharma, Inc. (NS Pharma) announced a change of leadership within its Commercial division. Effective September 9, 2024, Donald Foy – who had previously served as national sales director – was appointed to the role of vice president, Commercial. Jennifer Tamberino – who had been Regional Business Director, East, National Sales – was promoted to backfill Foy’s former position as national sales director.
“It is with incredible excitement that we announce Don’s promotion to lead our sales, marketing, market access, operations and patient services departments as our new head of Commercial at NS Pharma,” said NS Pharma President Yukiteru Sugiyama, Ph.D. “With Don and Jennifer at the helm, we are well- positioned to execute our plans for growth in the rare disease space in the United States. Our new leadership structure is designed to foster industry collaboration and company innovation from the top down.”
Foy replaces outgoing executive Gardner Gendron, who held the position for five years and oversaw the launch and commercialization of viltolarsen (VILTEPSO) for the treatment of Duchenne muscular dystrophy (Duchenne), a rare muscle-wasting neurological disorder.
Prior to joining NS Pharma, Foy served in leadership roles for 17 years in the pharmaceutical industry, with more than 24 years of experience both in sales and in cross-functional positions supporting a diverse set of stakeholders.
Tamberino has a four-year tenure with NS Pharma demonstrating effective leadership and business acumen.
NEWS RELEASE
NS Pharma currently has one commercial product, VILTEPSO, with several others in its pipeline for the treatment of Duchenne and, separately, the treatment of eosinophilic granulomatosis with polyangiitis (EGPA), a form of vasculitis. It is also slated to market deramiocel (CAP-1002), which is being developed by Capricor Therapeutics for the treatment of Duchenne cardiomyopathy.
About VILTEPSO® (Viltolarsen) Injection
Prior to its approval in the U.S. in August 2020, VILTEPSO was granted Priority Review as well as Rare Pediatric Disease, Orphan Drug and Fast Track Designations. In March 2020, VILTEPSO was approved in Japan for the treatment of patients with Duchenne who are amenable to exon 53 skipping therapy. Prior to its approval in Japan, VILTEPSO was granted the SAKIGAKE designation, orphan drug designation, and designation of Conditional Early Approval System.
Indication
VILTEPSO is indicated for the treatment of Duchenne in patients who have a confirmed mutation of the Duchenne gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VILTEPSO. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Important Safety Information
Warnings and Precautions: Kidney toxicity was observed in animals who received viltolarsen. Although kidney toxicity was not observed in the clinical studies with VILTEPSO, the clinical experience with VILTEPSO is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides. Kidney function should be monitored in patients taking VILTEPSO. Serum creatinine may not be a reliable measure of kidney function in patients with Duchenne.
Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be
measured before starting VILTEPSO. Consider also measuring glomerular filtration rate before starting VILTEPSO. During treatment, monitor urine dipstick every month, and serum cystatin C and urine protein-to-creatinine ratio every three months.
Urine should be free of excreted VILTEPSO for monitoring of urine protein. Obtain urine either prior to VILTEPSO infusion, or at least 48 hours after the most recent infusion. Alternatively, use a laboratory test that does not use the reagent pyrogallol red, which has the potential to generate a false positive result due to cross reaction with any VILTEPSO in the urine. If a persistent increase in serum cystatin C or proteinuria is detected, refer to a pediatric nephrologist for further evaluation.
Adverse Reactions: The most common adverse reactions include upper respiratory tract infection, injection site reaction, cough, and pyrexia.
To report an adverse event, or for general inquiries, please call NS Pharma Medical Information at 1-866-NSPHARM (1-866-677-4276)
For more information about VILTEPSO, see full Prescribing Information.
About NS Pharma, Inc.
NS Pharma, Inc., is a wholly owned subsidiary of Nippon Shinyaku Co., Ltd. NS Pharma is a registered trademark of the Nippon Shinyaku group of companies. For more information, please visit nspharma.com.
U.S. Media Contact: